Chapter 16 Discovery of the Electron. Balanced is the key word that is used to describe equilibrium situations.
This extends from Newtons first law of motion.
. A good example of this phenomenon is the reaction in which NO 2 dimerizes to form N 2 O 4. System as it came to thermal equilibrium Suppose 01 kg ice at 0oC 273K is in 05kg water at 20oC 293K. For each of the following reactions use Figure 164 to predict whether the equilibrium lies predominantly to the left or to the right.
C Lastly calculate the net work on the cart. AP Physics 1- Momentum Impulse and Collisions Practice Problems ANSWERS FACT. Assumptions are as in Figure A2.
2 NO 2 g N 2 O 4 g This reaction is favored by enthalpy because it forms a new bond which makes the system more stable. The defining quality of diffusion is that it takes place because the diffusing substance exists in a higher concentration in one place and a lower concentration in another. The Physics Classroom serves students teachers and classrooms by providing classroom-ready resources that utilize an easy-to-understand language that makes learning interactive and multi-dimensional.
Chapter 14 Worksheet 1 ws141 Chemical Equilibrium the Equilibrium Constant Keq and the Reaction Quotient Q As a reaction proceeds in a. For the chemical equilibrium A 2B 2C the value of the equilibrium constant K is 10. Chapter 20 Entropy and the Second Law of.
Determine the value of the equilibrium constant Kc for the reaction. These scales indicate the equilibrium commitment to future warming caused by emissions from 1990 through 2030. Objects at equilibrium must have an acceleration of 0 mss.
Written by teachers for teachers and students The Physics Classroom provides a wealth of resources that meets the varied needs of both students and teachers. Here is a set of practice problems to accompany the Derivatives of Trig Functions section of the Derivatives chapter of the notes for Paul Dawkins Calculus I. 2A 4B 4C K.
To determine equilibrium warming in 2030 due to continued emissions of CO 2 at the 1990 level find the point on the curve labeled CO 2 that is vertically above 0 percent change on the bottom scale. Characteristics of Equilibrium VIDEO. Derive an equation for the speed of the box when the spring returns to its equilibrium position.
S k ln W. Closed system the concentrations of reactants and products change until at. Equilibrium Constant In the equilibrium region the concentrations of products and reactants are related in an equation called the equilibrium constant expression In the 2butene case cis trans at 400 C K c is called the equilibrium constant.
A taxes subsidies Increase or decrease. I bring thirty-two years of full-time classroom chemistry teaching experience and tens of thousands of hours of one-on-one chemistry tutoring across the globe to a seventeen year writing career that includes several best-selling international award-winning chemistry books and a burgeoning. But having an acceleration of 0 mss.
At equilibrium the concentration of NO was found to be 0062 M. Le Ch telier s Principle Example 2. The textbook definition of diffusion is the movement of molecules or particles from an area of higher concentration to an area of lower concentration continuing until equilibrium is reached.
Entropy and Equilibrium VIDEO DETAILED NOTES ON FACTORS AFFECTING EQUILIBRIUM GRAPHS INVOLVING EQUILIBRIUM Unit_2 notesP24_40 DETAILED NOTES ON LeCHATELIERS PRINCIPLE EQUILIBRIUM CONSTANT Keq Unit2NotesP41-61. Equilibrium is the price at which the quantity demanded by consumers is equal to the quantity thats supplied by suppliers. Le Ch telier s Principle Example 1 VIDEO.
Supply decreases curve shifts inward or left Equilibrium After P2 Q2 price - t Quantity - Equilibrium Before PI QI. Readily while the weaker one tends to retain its proton. Included in this list are printable pdf chemistry worksheets so you can practice problems and then check your answers.
A 2B 2C K 10 a. ΔS total ΔS melt. Thus the equilibrium favors the direction in which the proton moves from the stronger acid and becomes bonded to the stronger base.
Alphabetical Index of Chemistry Problem Types. Equilibrium After P2 Q2 Price- Quantit Equilibrium Before PI QI Change Tax on manufacturer Supply or Demand first. Moving at a constant velocity determine the work done by the force of friction.
What is the value of the equilibrium constant for the following reaction. Thus the net force is zero and the acceleration is 0 mss. As a result the equilibrium constant must depend on the temperature of the reaction.
Consider the following reaction N2O4 g 2 NO2 g. By taking the freezing point constant for water as 186 from Table and then substituting the values into the equation for freezing point depression you obtain the change in freezing temperature. The product of mass and velocity is a vector quantity known as momentum The equation for linear momentum is 𝑚𝑣 and has the units kg 𝑚 which can also be.
Initially a mixture of 0100 M NO 0050 M H2 0100 M H2O was allowed to reach equilibrium initially there was no N2. Or in other words two objects where heat isnt. If an object is at equilibrium then the forces are balanced.
Entropy is the Boltzmann constant k 138x10-23 JK times the natural log of W. Im a true chemistry freelancer and Subject Matter Expert SME. For the chemical equilibrium A 2B 2C the value of the equilibrium constant K is 10.
For K cspecies are 1expressed as M Moles L. The reaction is not favored by entropy. Thomson was the Cavendish professor of Experimental Physics at Cambridge University and director of its Cavendish Laboratory from.
Δ T f 186Cm 0365 m 068C. Here is a set of practice problems to accompany the Computing Limits section of the Limits chapter of the notes for Paul Dawkins Calculus I course at Lamar University. AP Physics 1- Work Energy Power Practice Problems ANSWERS FACT.
Thomson Joseph John Thomson J. Include the steps outlined in class as part of your solution large clear Free Body Diagram resolve forces into components use hatch marks to indicate equal forces apply Newtons 2nd Law to each direction. When either demand or supply changes however the equilibrium price and.
See photo at American Institute of Physics is widely recognized as the discoverer of the electron. Worksheet 44 Equilibrium Applications of Newtons Laws Solve the following problems on another sheet of paper. If 134 moles each of Brp g and C12 g are introduced in a container which has a volume Of 110 liters and allowed to reach equilibrium at 205 0 C what would be.
You may also browse chemistry problems according to the type of problem. The amount of. Thermal equilibrium is defined as the state in which two objects connected by a permeable barrier dont have any heat transfer between them.
At 205 oc the equilibrium constant Kc is 0143.
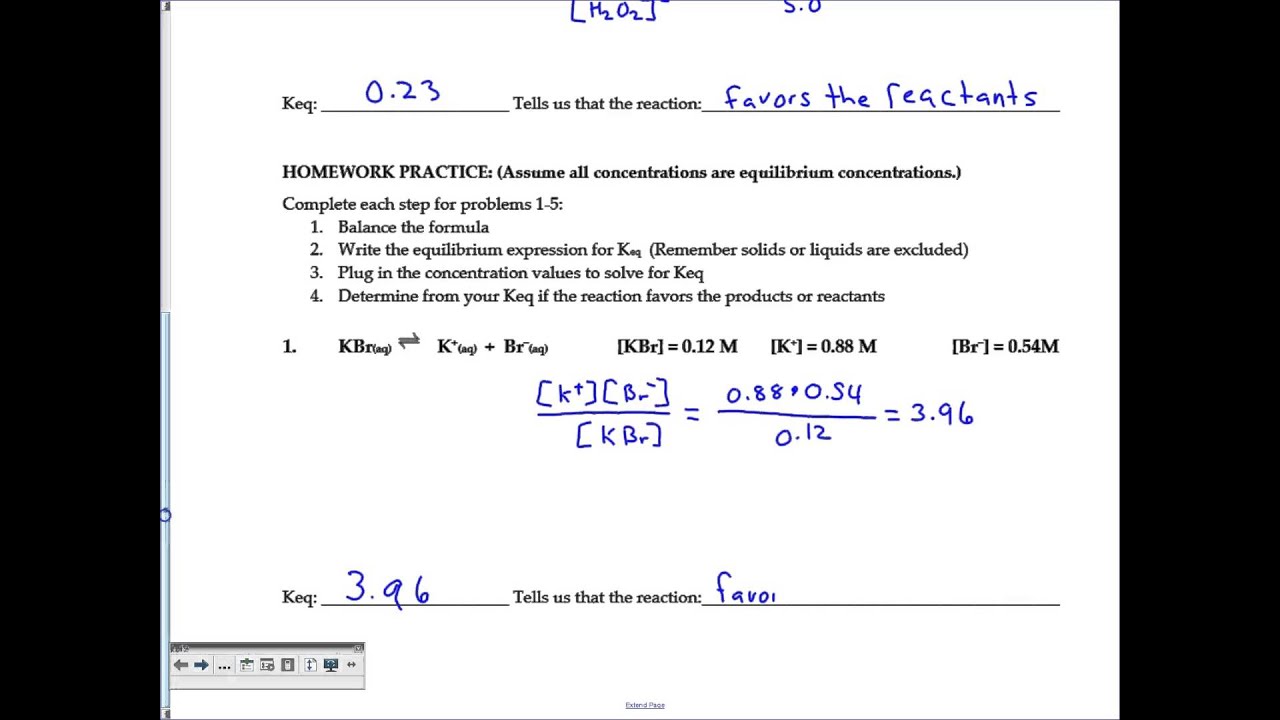
02 Equilibrium Constant Worksheet Key Youtube

Solved Keq And Ice Problems Worksheet 1 Calculate The Chegg Com

Chemistry 12 Worksheet Keq Level 3 Questions
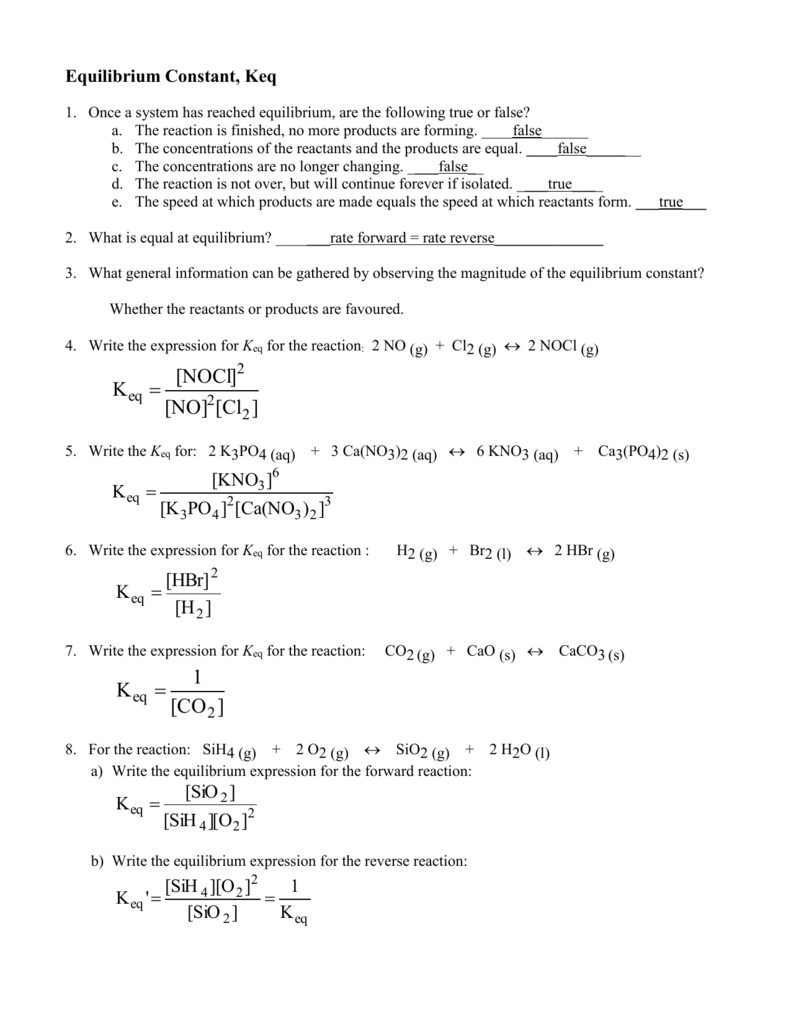
Equilibrium Worksheet Answers Sch4u1 Ccvi

Dr Baxley S Equilibrium Worksheet
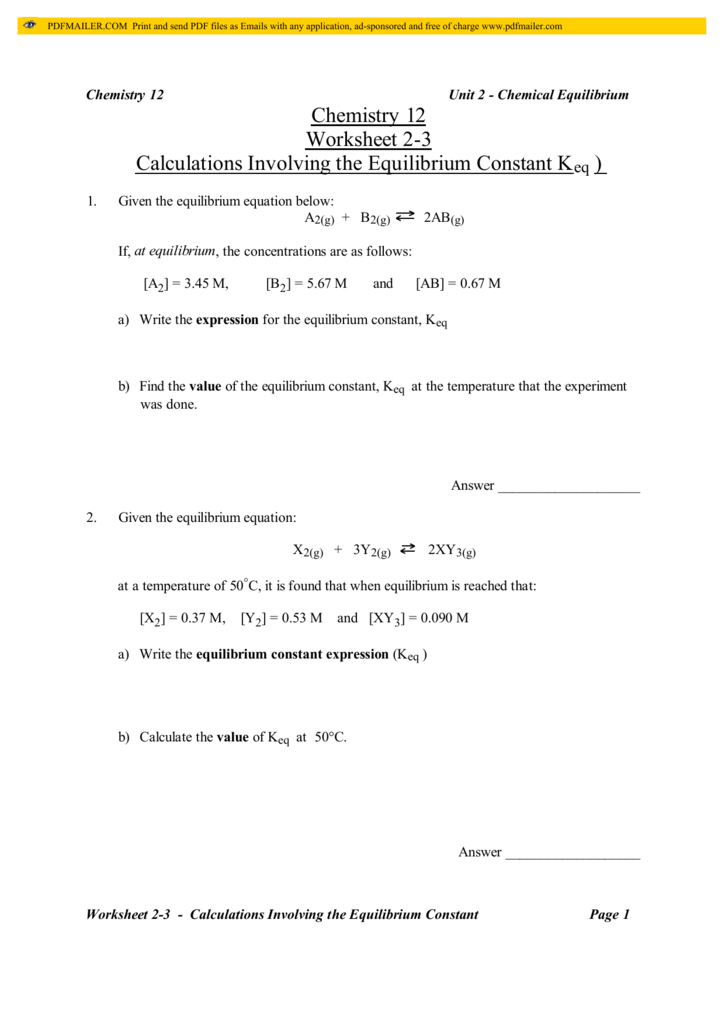
Chemistry 12 Worksheet 2 3 Calculations Involving The Equilibrium

The Equilibrium Constant Lesson Plans Worksheets

Solved Keq And Ice Problems Worksheet Calculate The Chegg Com

Equilibrium Worksheet Solutions Final Name Equilibrium Worksheet Solutions Complete The Following Questions On A Separate Piece Of Paper 1 Write The Course Hero

Solved Please Show Your Work Calculations Thank You Very Chegg Com

Quiz Worksheet Rate Constant Equilibrium Constant Study Com

Quiz Worksheet Dynamic Equilibrium Study Com

Equilibrium Constant Expression Worksheet

Equilibrium Constant Homework Equilibrium Constant K Name Write The Expression For The Equilibrium Constant K For The Reactions Below I N2 G 3h2 G E Course Hero


ConversionConversion EmoticonEmoticon